
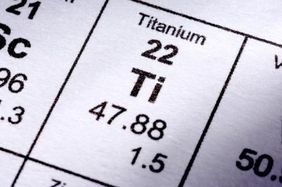
The average atomic mass of titanium will thus beĪvg. the average atomic mass Carbon has 3 natural occurring isotopes: Carbon-12 has a mass of 12.009 with a percent abundance of 98.85. The decimal abundances for these five isotopes will be: The average of the total mass of all an element's isotopes is called: a. Simply put, each isotope i will contribute to the average atomic mass of the element in proportion to its decimal abundance, which is simply the percent abundance divided by 100.Ī v e r a g e a t o m i c m a s s = ∑ i m a i × a b u n d a n c e o f i Now, the average atomic mass of titanium is calculated by taking the weighted average of the atomic masses of its stable isotopes. O-16 The average atomic is closest to 16 amu. So technically, both atomic mass and average atomic mass are atomic masses, but one represents a single atom, and the other represents the average of the isotopes.When the problem doesn't provide you with the actual atomic mass of an isotope, m a , you can use its mass number, A, as an approximation of its atomic mass. Using Oxygens average atomic mass from the periodic table, explain which isotope is the most abundant and how you know. average atomic mass of oxygen to calculate the average atomic mass of X. It is the abundance of isotopes of an element found naturally, expressed in percentages. Why is this 0.26 Distinguish between the two terms atomic. Its unit is also amu.īut the average atomic mass depends on one more critical aspect, the isotopic abundance. The average atomic mass expresses the atomic mass of elements with isotopes. Boron exists in two isotopes, boron-10 and boron-11. Calculate the atomic mass of copper if copper-63 is 69.17 abundant and copper-65 is 30.83 abundant. Isotopes became the reason for calculating the average atomic masses, as we must consider an element's isotopes. What is the average atomic mass of titanium Answer: 47.92 amu 6 6 6. Then came isotopes, the atoms that differ slightly in atomic masses due to the varying number of neutrons in their nucleus. The mass of 0.17 m3 volume titanium can be. The unit of atomic mass is non-SI, amu (atomic mass unit). For chemistry students and teachers: The tabular chart on the right is arranged by Atomic mass (weight). The liquid may be ethyl alcohol Example 3: Density to Calculate Volume Mass. This calculation gives us the mass of a single atom of an element. Example/Practice: Titanium has five common isotopes: 46Ti (8.0), 47Ti (7.8), 48Ti (73.4), 49Ti (5.5), 50Ti (5.3). But we can figure it out by adding up the number of protons and neutrons in the nucleus of an atom. It is the mass of a single atom of that element.Įxperimentally it is calculated by mass spectrometry (an analytical technique used to measure the mass-to-charge ratio of ions). How many atoms are in 3.56 moles of copper 6. How many molecules are contained in 6.53 mol of nitrogen gas, N2 3.

We have been familiar with the atomic mass of an element since we started learning about elements and their atoms. What is the average atomic mass of titanium 2.


 0 kommentar(er)
0 kommentar(er)
